There are three different types of Alcohol molecules –
1. Primary Alcohol; is when the -OH is at the end of the chain.
2. Secondary Alcohol; is when the -OH group is attached to a carbon atom bonded to two alkyl groups.
3. Tertiary Alcohol; is when the -OH group is attached to a carbon atom bonded to three alkyl groups.
Combustion and Oxidation of alcohols
In a good supply of oxygen, alcohols burn completely to form carbon dioxide and water –
C2H5OH(l) + 3O2 – 2CO2(g) + 3H2O(l)
Oxidation of alcohols
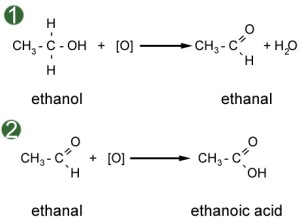
- Primary alcohol
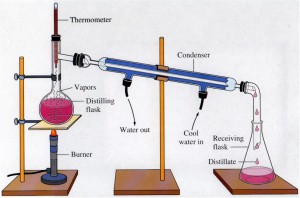
Figure 1 shows how alcohol forms aldehyde under distillation*.
Figure 2 shows how alcohol froms carboxylic acid under reflux**.
**Reflux is the continual boiling and condensing of a reaction mixture to ensure that the reaction takes place without the contents of the flask boiling dry.
*Distillation apparatus
**Reflux apparatus
- Secondary alcohol
Secondary alcohols are oxidised by acidified dichromate ions to produce ketones.
- Tertiary alcohol
Tertiary alcohols are resistant to oxidation as the carbon atom is already bonded to three other alkyl groups.