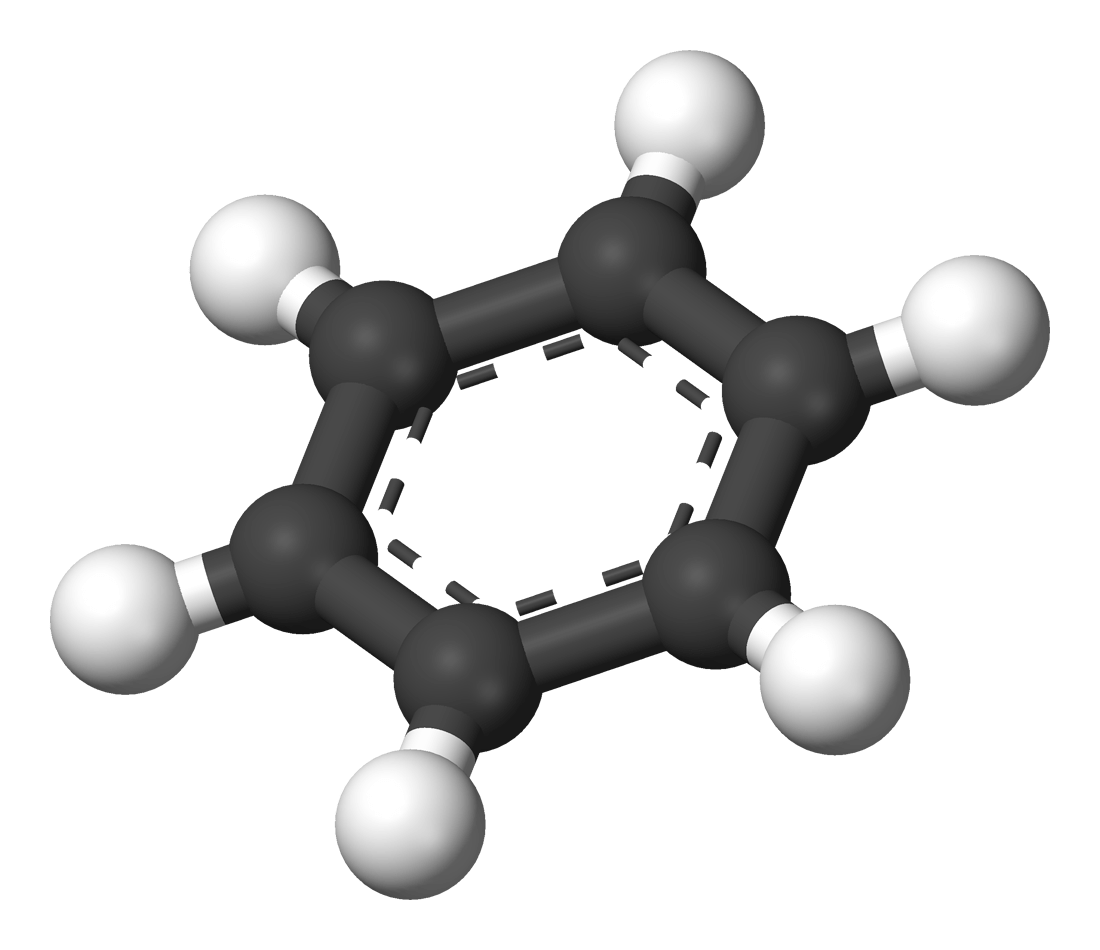
Electrophilic substitution
 – electrophilic substitution by nitration
– electrophilic substitution by nitration
- The electrophlie is attracted to an electron rich ring, and the electrophile accepts a pair of the pi-electrons from the delocalised ring to form a covalent bond.
- The H is substituted by an electrophile, and the delocalised pi-electron cloud has been disrupted and the intermediate is less stable.
- The unstable intermediate repidly loses the hygrogen as a H+
Hydrogenation of benzene

Alkylation of benzene

AlCl3 acts as catalyst as it causes the Cl2 to split through heterolytic fission. Once the H+ is substituted, it takes the extra Cl molecule forming HCl.
Acylation of benzene

Sulfonation of benzene







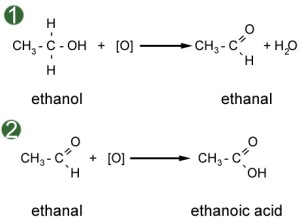
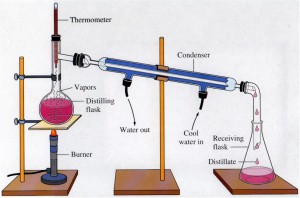





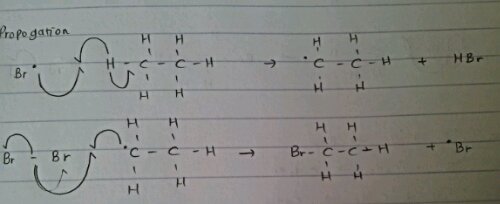
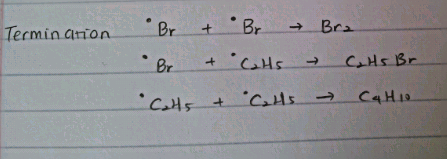
.png/350px-Heterolysis_(Chemistry).png)










 etc.
etc.